Background: Iron deficiency anemia (IDA) is common in the pediatric population with high risk factors such as nutritional deficiency, inflammatory bowel disease (IBD) and other bowel inflammatory disorders, menorrhagia, blood loss, poor absorption and anemia of chronic disease. Intravenous (IV) iron supplementation has become a more desirable mode of treatment in patients with moderate to severe anemia and in patients who are either unresponsive to or have undesirable side-effects secondary to oral iron. Iron sucrose and Iron dextran have been traditionally used in pediatrics as they both are FDA approved for use in this population. Ferric carboxymaltose (FCM) has only been FDA approved for use in adults however is currently used in pediatrics as well. One of the major advantages of Ferric carboxymaltose is the ease of dosing and efficacy. Though FCM was approved for adults in 2013 and there have been no safety concerns, it is not yet FDA approved for pediatric patients despite a few pediatric studies demonstrating its safety and efficacy (Laass, et al., 2014; Powers et al., 2017; Tan et al., 2017, Carman et al., 2019). The purpose of this study is to examine the utilization of different IV iron formulations in a large pediatric hospital as well as evaluate the safety and efficacy of ferric carboxymaltose in comparison to other IV iron formulations. At this time, we present data regarding the use of different forms of IV iron.
Methods: This is a retrospective chart review study of all patients who met inclusion criteria in a large pediatric hospital who received Iron dextran, Iron sucrose, and/or FCM between the dates of 8/1/2018 through 9/30/2019. Anonymized data from eligible patients was entered into a secure electronic database. Once our population of interest was isolated, based on the proposed criteria, we reviewed charts individually and collected data including demographics and details about each IV iron administration. Demographic data encompassed race and gender. We also recorded the patients' underlying diagnosis (or diagnoses) contributing to iron deficiency anemia. In addition to compiling demographic data, we also wanted to analyze the trend of IV iron usage in our institution. This was done by tallying the number of each type of IV iron infusion monthly for the allotted time period.
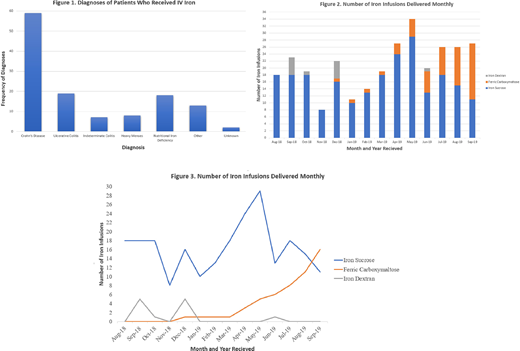
Results: A total of 120 patients met inclusion criteria and were included in this study with details regarding diagnosis in Figure 1. Fifty-six (46.7%) patients were male and 64 (53.3%) were female. We also analyzed the underlying diagnoses leading to IDA of patients who received IV iron infusions. Most patients had an underlying IBD diagnosis (Crohn's Disease 49.2%, Ulcerative colitis 15.8%, and Indeterminate colitis 5.8% of all included patients). Additional diagnoses included 18 patients (15%) with nutritional IDA and 8 patients (6.7%) with heavy menses. Examples of "other" diagnoses are blood loss secondary to immune thrombocytopenia, short bowel secondary to complex gastroschisis, gastrointestinal bleed secondary to Helicobacter pylori, short bowel secondary to bowel resection due to graft versus host disease after hematopoietic stem cell transplant, TMPRSS6 mutation, protein losing enteropathy, short bowel secondary to midgut volvulus and intestinal atresia, among other diagnoses.
We also analyzed the overall usage of IV iron in our institution during this same time period. The number of IV iron infusions has steadily increased since August 2018. The average number of IV iron infusions was 18 per month in 2018 and 22.67 per month in 2019. As shown in figure 3, the utilization of iron dextran has decreased over time. The graph also displays that the usage of FCM at our institution continues to increase as time progresses.
Conclusion: Analysis of demographic data reveals that an underlying gastrointestinal diagnosis is the most common reason for receiving IV iron likely due to decreased absorption of enteral iron. Our data has also shown that the overall usage of IV iron is increasing in the pediatric population as well as specifically FCM. This study is the first retrospective pediatric review comparing the utilization of different IV iron formulations including FCM. Preliminary data demonstrates an increase in hemoglobin after treatment with FCM, however further analysis of the data is ongoing.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal